In areas where the drinking water contains unsafe levels of arsenic, the immediate concern is finding a safe source of drinking water. There are two main options: Finding a new safe source or removing arsenic from the contaminated source. If an arsenic safe water source cannot be established, the short-term goal is to reduce arsenic levels.There are several methods available for removal of arsenic from water. The following important methods are discussed below:
- Oxidation
- Coagulation, precipitation and filtration
- Adsorption (sorptive filtration)
- Ion exchange
- Membrane techniques

Most arsenic removal technologies are most effective in removing the pentavalent form of arsenic (As(V), arsenate), since the trivalent form (As(III), arsenite) is predominantly non-charged below pH 9.2. Thus arsenate is much less mobile than arsenite, as it tends to co-precipitate out with metallic cations or to adsorb onto solid surfaces. Therefore, many treatment systems include an oxidation step to convert arsenite to arsenate. Arsenite can be oxidised by oxygen (O2), hypochlorite (HClO), permanganate (HMnO4) and hydrogen peroxide (H2O2). Atmospheric oxygen is the most readily available oxidising agent and many treatment processes prefer oxidation by air. However, air oxidation of arsenic is a very slow process and can take weeks for oxidation (PIERCE & MOORE 1982). Air oxidation of arsenite can be catalysed by bacteria, strong acidic or alkali solutions, copper, powdered activated carbon and high temperature (EDWARDS 1994).
Oxidation with the oxygen contained naturally in the air during collection and subsequent storage in houses may cause a reduction in arsenic concentration in stored water, which is also known as passive sedimentation. For passive sedimentation, the water needs to be stored for a sufficiently long time allowing the exchange of oxygen from the air to the water. Arsenic reduction by plain sedimentation appears to be dependent on water quality, particularly the presence of precipitating iron in water. High alkalinity and presence of iron in the tube wells water increase arsenic removal by storage.
In-situ oxidation of iron and arsenic in the aquifer has been tested under the DANIDA (Ministry of Foreign Affairs of Denmark) Arsenic Mitigation Pilot project in Bangladesh. The process technology is to extract water from a tube well to let it oxygenate at the air with atmospheric oxygen. Oxygenated water is then allowed to run back into the iron and arsenic contaminated aquifer through the same tube well. This allows forming coating of iron hydroxide on sand grains around the strainer of the well. Water re-collected from the well will be significantly reduced in arsenic and iron.
Coagulation and filtration with metal salts and lime followed by filtration is the most heavily documented method of arsenic removal from water.In the process of coagulation, arsenic is removed from solution through three mechanisms (EDWARDS 1994).
- Precipitation:the formation of insoluble compounds.
- Co-precipitation:the incorporation of soluble arsenic species into a growing metal hydroxides phases (e.g. co-precipitation with Fe(III);
- Adsorption:the electrostatic binding of soluble arsenic to external surfaces of the insoluble metal hydroxide.
Coagulation technology has been used since 1970 in northern Chile for removing arsenic from drinking water. This experience suggests that coagulation is an effective technology for the removal of arsenic. It is currently possible to reduce arsenic from 400 μg/L to 10 μg/L at a rate of 500 L/sec, assuming pH, oxidising and coagulation agents are strictly controlled (SANCHA 2006).
Coagulation-flocculation processes using alum, ferric chloride, or ferric sulphate are effective at removing arsenic. They are the most well known arsenic treatments and have been more extensively tested in both laboratory and field studies than other technologies (VU et al. 2009). When added into water, they dissolve under efficient stirring for one to few minutes. During this flocculation process, all kinds of micro-particles and negatively charged ions are attached to the flocs by electrostatic attachment. Arsenic is also adsorbed onto coagulated flocs. It can be removed partially by sedimentation,while filtration may be required to ensure complete removal of all flocs. Arsenic removal by coagulation is mainly controlled by pH and coagulation dose. Coagulation with ferric chloride works best at pH below 8 and Alum has a narrower effective range from pH 6 to 8 (JOHNSTON and HEIJNEN 2001).
The Bucket Treatment Unit (BTU) and the Stevens Institute technology
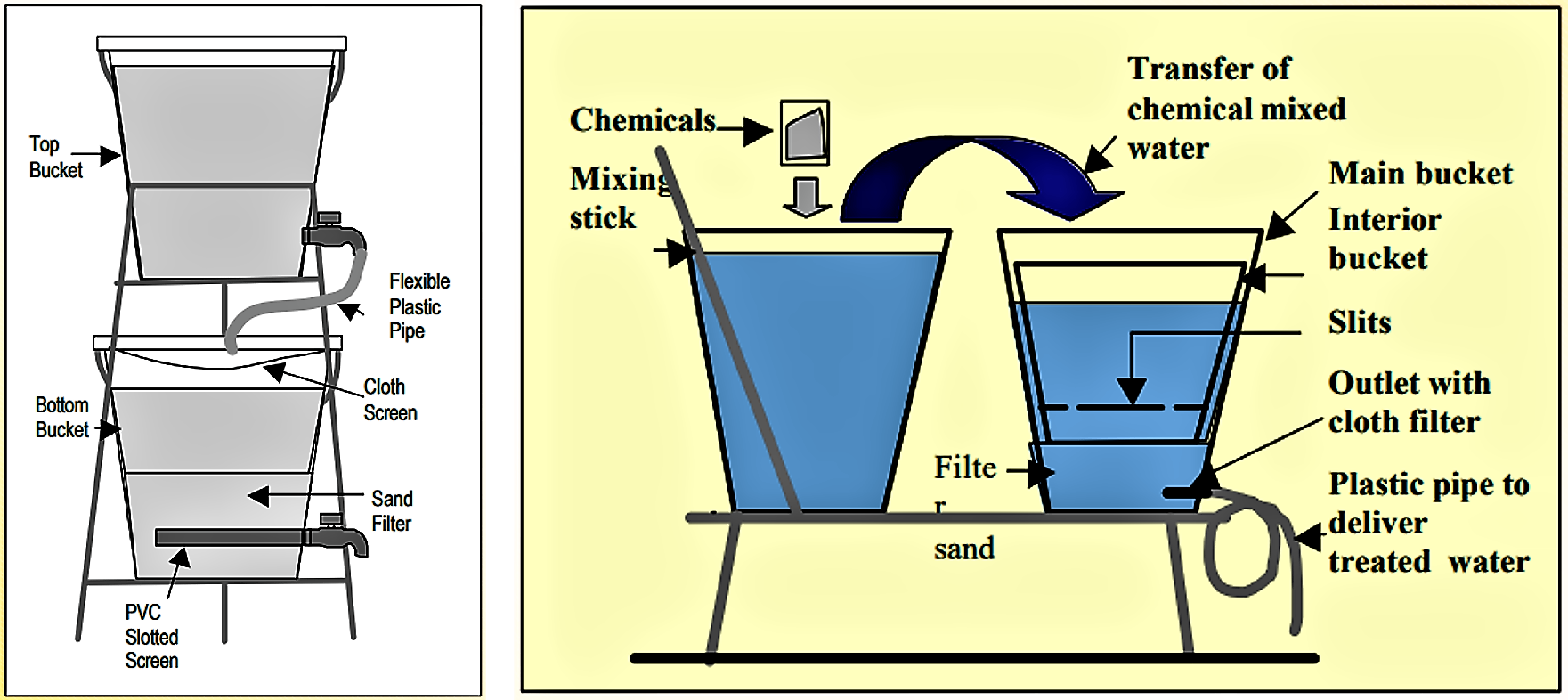
The Bucket Treatment Unit (BTU), developed by the DANIDA project in Bangladesh is based on the above-described coagulation and filtration process. It consists of two buckets, each 20 Litres, placed one above the other. Chemicals are mixed manually with arsenic contaminated water in the upper bucket and stirred with for 30 to 60 seconds. The chemical used includes aluminium, sulphate and potassium permanganate supplied in powder form. The water from the top bucket is then allowed to flow into the lower green bucket via plastic pipe and a sand filter box installed in the lower bucket.
The Stevens Institute technology is similar to the BTU method. It uses also two buckets, one to mix chemicals (iron sulphate and calcium hypochlorite supplied in packets) and the other to separate flocs by the processes of sedimentation and filtration (BAMWSP et al. 2001).
Use of naturally occurring iron present in groundwater
The use of naturally occurring iron present in groundwater is a promising method of removing arsenic by adsorption, meaning that there is no need for adding chemicals. The iron precipitates formed by oxidation of dissolved iron has been found to remove arsenic by coagulation, adsorption, precipitation and filtration, but also by oxidation. The efficiency of the units depends on arsenic and iron contents of water. It can be heightened by increasing the contact time between arsenic species and iron flocs.
Coagulation with lime
Water treatment by the addition of quick lime, CaO, or hydrated lime, Ca(OH)2 removes arsenic. Lime treatment is a process similar to coagulation with metal salt. The precipitated calcium hydroxide, Ca(OH)2 acts as a sorbing flocculent for arsenic. Excess of lime will not dissolve, but remains as a coagulant aid, which has to be removed along with precipitates through sedimentation and filtration process. It has been observed that the arsenic removal by lime is relatively low, usually between 40-70 %. The highest removal is achieved at pH 10.6 to 11.4 (AHMED 2001). Lime softening may be used as a pre-treatment to be followed by alum or iron coagulation.
Solar oxidation and precipitation of Fe(III)-oxides with adsorbed As(V)
SORAS is a simple method that uses irradiation of water with sunlight in PET- or other UV-A transparent bottle (see also SODIS) to reduce arsenic levels in drinking water. The SORAS method is based on two steps: the first step consists in photochemical oxidation (through the action of the solar UV light) of As (III) to As(V) followed by the second step consisting in precipitation or filtration of As(V) adsorbed on Fe(III)-oxides, which are either naturally present or added and kept in suspension by the addition of lime juice. It could be a water treatment method used at household level to treat small quantities of drinking water. Groundwater in Bangladesh naturally contains Fe (II) and Fe (III); therefore SORAS could reduce arsenic contents and would be available to everyone at virtually no cost (WEGELIN et al. 1999).
Several sorptive media like activated alumina, activated carbon, iron and manganese coated sand, kaolinite clay, hydrated ferric oxide, activated bauxite, titanium oxide, silicium oxide and many natural and synthetic media have been reported to remove arsenic from water. The efficiency of sorptive media depends on the use of oxidising agents as aids to provoke the sorption of arsenic on the media.
Activated alumina
Activated alumina (Al2O3) has a good sorptive surface, in the range of 200-300 m2/g. The large surface area gives the material a very large area for adsorption of arsenic. When water passes through a packed column of activated alumina, the impurities including arsenic present in water are adsorbed on the surfaces of activated alumina grains. Eventually, the column becomes saturated, first at its upper zone and later downstream towards the bottom end, and finally the column gets totally saturated. Regeneration of saturated alumina is carried out by exposing the medium to 4% caustic soda (NaOH) either in batch or by flow through the column resulting in highly arsenic-contaminated caustic wastewater. Arsenic removal by activated alumina is controlled by pH and the arsenic content of water. The efficiency drops as the point of zero charge is approached and at pH 8.2 where the surface is negatively charged, the removal capacities are only 2-5% of the capacity at optimal pH (CLIFFORD 1990). Some examples of activated alumina based sorptive media are: the “BUET Activated Alumina”, the “Alcan Enhanced Activated Alumina” and the “Apyron Arsenic Treatment Unit”.
Granular ferric hydroxide
Granular ferric hydroxide is also used for the adsorptive removal of arsenate, arsenite and phosphate from water. Granular ferric hydroxide reactors are fixed bed adsorbers operate like a conventional filter with a downward flow of water. The water containing high dissolved iron and suspended matters should be aerated and filtered through a gravel/sand bed as a pre-treatment to avoid clogging of the adsorption bed.
Hydrous cerium oxide
Hydrous cerium oxide is also a good adsorbent; laboratory test and field-testing of the materials at several sites showed that the absorbent is highly efficient in removing arsenic from groundwater.
Iron coated sand and brick chips
Iron coated sand and iron coated brick chips are effective in removing both As(III) and As(V). The “Shapla arsenic filter” is an example of a household arsenic removal filter based on iron coated brick chips developed and promoted by the International Development Enterprises (IDE). The brick chips are treated with ferrous sulphate solution for iron coating. The water collected from contaminated tube wells passes through the filter media placed in earthen container having a drainage system underneath.
Household-level arsenic filters
Some filters like the SONO 3 KALSHI, the KanchanTM or the SAFI arsenic filter use zerovalent iron fillings (solid iron), sand, brick chips and wood coke for removing arsenic, and other trace metals from ground water (see also arsenic filters). Arsenic is removed by adsorption on the mixture of partly oxidised zerovalent iron filling and sand.
The KanchanTM filter was developed by Massachusetts Institute of Technology (MIT), the Environment and Public Health Organisation (ENPHO) and the Rural Water Supply and Sanitation Program (RWSSSP) of Nepal. It combines slow sand filtration and adsorption on iron hydroxide and is efficient in removing arsenic, pathogens, iron, turbidity, odour and some other contaminants in drinking water. The filter is made up of a concrete or plastic box that is filled with layers of sand and gravel much like a biosand filter. On the top of the filter, as the first stage, a layer of 5 kg of iron nails is installed. These nails when exposed to air and water rust very quickly producing ferric hydroxides particles, which is an excellent absorbent of arsenic. When arsenic-containing water is poured into the filter, surface complexation reaction occurs, and arsenic is rapidly adsorbed onto the surface of the ferric hydroxide particles. The arsenic loaded iron particles are then flushed into the sand layer below. Because of the very small pore space in the fine sand layer, the arsenic loaded iron particles will be trapped in the top few centimetres of the fine sand layer. As a result, arsenic is effectively removed from the water.
The SAFI filter is an adapted ceramic candle filter that works by the principles of adsorption and filtration on chemically treated active porous composites materials of the candle. The filter is made of composite porous materials like kaolinite and iron oxide on which hydrated ferric oxide is deposited by sequential chemical and heat treatment. The oxy-hydroxides of iron, aluminium and manganese are involved in the removal of arsenic, iron and bacteria.
Ion exchange is similar to that of activated alumina; just the medium is a synthetic resin of better-defined ion exchange capacity. The synthetic resin is based on a cross-linked polymer skeleton, called the matrix. The charged functional groups are attached to the matrix through covalent bonding and fall into acidic, weakly acidic, strongly basic and weakly basic groups (CLIFFORD 1990). The ion exchange process is less dependent on pH of water. Arsenite, being uncharged, is not removed by ion exchange process. Hence, pre-oxidation of As(III) to As(V) is required for removal of arsenite by ion exchange process but the excess of oxidant often needs to be removed before the ion exchange in order to avoid the damage of sensitive resins. As the resin becomes exhausted, it needs to be regenerated. Ion exchange resins can be easily regenerated by washing with a NaCl solution.
Synthetic membranes are used to eliminate many contaminants from water including pathogens, salts and various metal ions. Usually, two types of membrane filtration are used: low-pressure membranes such as microfiltration and ultrafiltration and high-pressure membranes such as nanofiltration and reverse osmosis. Arsenic removal by membrane filtration is independent of pH and presence of other solutes but adversely affected by presence of colloidal matters. Iron and manganese can also lead to scaling and membrane fouling. The membrane once fouled by impurities in water cannot be backwashed. The water having high concentrations of suspended solids requires pre-treatment for arsenic removal by membrane techniques to avoid clogging.
Other potential approaches would include the use of phytoremediation or rhizofiltration. Phytoremediation use green plants to remove pollutants from the environment and render them non available. Rhizofiltration is defined as the use of plant roots to adsorb, absorb, concentrate and precipitate metal from solution. Several plants species are known to accumulate metals from environment including water hyacinth (Eichhornia crassipes), which grows in waterways in many parts of the world and has been used as a model system for studying the uptake of metal ions by aquatic plants. Biomaterial produced from dried water hyacinth roots can provide a simple, effective and yet cheap method for removing arsenic from contaminated water. It has been found that more than 93% of arsenite and 95% of arsenate were removed within 60 minutes of exposure to a powder produced from dried roots. (RMALLI et al. 2005). Some researchers have shown that certain types of bacteria can play an important role in catalysing biological arsenic removal processes.
All the arsenic treatment technologies ultimately concentrate arsenic in sorption media, sludge or liquid media and indiscriminate disposal of these may lead to environmental pollution. Hence, environmentally safe disposal of sludge, saturated media and liquid wastes rich in arsenic is of high concern. Experiments were conducted to assess transformation of arsenic from aqueous solutions in the presence of cow dung. Some studies suggested that bio-chemical (e.g., bio-methylation) process in the presence of fresh cow-dung may led to significant reduction of arsenic from arsenic rich treatment wastes (ALI et al. 2001). Another option would be to blend the arsenic contaminated material into stable waste or engineering materials such as glass, bricks, concrete or cement blocks. However, there is also possibility of air pollution or water pollution downstream of kilns burning bricks containing arsenic contaminated sludge due to volatilisation of arsenic during burning at high temperature.
An Overview of Arsenic Removal Technologies in Bangladesh and India
The most common technologies for arsenic removal utilise conventional processes like oxidation, co-precipitation, adsorption, ion exchange and membrane techniques. The paper gives a short review of all these technologies, which are used in Bangladesh and India.
AHMED, M. F. (2001): An Overview of Arsenic Removal Technologies in Bangladesh and India. In: BANGLADESH UNIVERSITY OF ENGINEERING AND TECHNOLOGY ; UNITED NATIONS UNIVERSITY (2001): Technologies for Arsenic Removal from Drinking Water. Dhaka: 251-269. URL [Accessed: 21.05.2019]Treatment of Arsenic Contaminated Water
Arsenic Contamination in Southeast Asia Region: Technologies for Arsenic Mitigation
PowerPoint presentation in PDF version, which includes information on arsenic contamination globally and in Southeast Asia with an overview on existing arsenic mitigation water supply technologies.
AHMED, M. F. (n.y): Arsenic Contamination in Southeast Asia Region: Technologies for Arsenic Mitigation. (= PPT Presentation ). Dhaka: Bangladesh University of Engineering and Technology URL [Accessed: 14.05.2012]Development of Low-cost Technologies for Removal of Arsenic from Groundwater
This paper includes a performance evaluation of three alternative arsenic removal technologies/systems in the laboratory in order to determine their suitability for the development of a low-cost arsenic removal unit.
ALI, M. A. BADRUZZAMAN, A.B.M. JALIL, M.A. HOSSAIN, M. D. HUSSAINUZZAMAN, M. M. BADRUZZAMAN, M. MOHAMMAD, O.I. AKTER, N. (2001): Development of Low-cost Technologies for Removal of Arsenic from Groundwater. Dhaka: Bangladesh University of Engineering and Technology, Department of Civil Engineering URL [Accessed: 20.05.2019]Rapid Assessment of Household Level Arsenic Removal Technologies, Phase-I and Phase-II - Final Reports
Ion Exchange and Inorganic Adsorption
Chemistry of Arsenic Removal during Coagulation and Fe-Mn Oxidation
Adsorption of Arsenite and Arsenate on amorphous iron hydroxide
Safe Water Technology for Arsenic Removal. Technologies for arsenic Removal from Drinking Water
This paper describes some safe water technologies for arsenic removal, with special reference to experiences gained from field level application.
JOHNSTON, R. HEIJNEN, H. (2001): Safe Water Technology for Arsenic Removal. Technologies for arsenic Removal from Drinking Water. In: BANGLADESH UNIVERSITY OF ENGINEERING AND TECHNOLOGY ; UNITED NATIONS UNIVERSITY (2001): Technologies for Arsenic Removal from Drinking Water. Dhaka: 1-21. URL [Accessed: 14.05.2012]Household Sand Filters for Arsenic Removal
A Biomaterial Based Approach for Arsenic Removal from Water
In this paper, it is shown that a biomaterial produced from dried water hyacinths roots, a plant that is found in abundant supply in many parts of the world, can provide a simple, effective and yet cheap method for removing arsenic from contaminated water.
RMALLI, S. W. ; HARRINGTON, C. F. ; AYUB, M. ; HARIS, P. I. (2005): A Biomaterial Based Approach for Arsenic Removal from Water. In: Journal of Environmental Monitoring : Volume 7 , 279-282. URL [Accessed: 12.01.2011]Review of Coagulation Technology for Removal of Arsenic: Case of Chile
This paper presents a summary of the process, concepts and operational considerations for the use of coagulation technology for removal of arsenic in Chile.
SANCHA, A. M. (2006): Review of Coagulation Technology for Removal of Arsenic: Case of Chile. In: Journal of Health, Population and Nutrition: Volume 24 , 267-272. URL [Accessed: 14.05.2012]Review of Arsenic Removal Technologies for Contaminated Groundwater
This article summarises the technologies currently being investigated to remove arsenic from drinking waters, with a special focus on developing and third-world countries where the problem is exacerbated by flooding and depressed economic conditions.
VU, K.B. KAMINSKI, M.D. NUNEZ, L. (2009): Review of Arsenic Removal Technologies for Contaminated Groundwater. Argonne: University of Chicago - Argonne National Laboratory URL [Accessed: 12.01.2011]SORAS - a simple arsenic removal process
This paper gives an overview on SORAS (Solar oxidation and removal of arsenic) with laboratory and field test results.
WEGELIN, M. GECHTER, D. HUG, S. MAHMUD, A. MOTALEB, A. (1999): SORAS - a simple arsenic removal process. Duebendorf: Eawag/SANDEC URL [Accessed: 20.05.2019]Development of Low-cost Technologies for Removal of Arsenic from Groundwater
This paper includes a performance evaluation of three alternative arsenic removal technologies/systems in the laboratory in order to determine their suitability for the development of a low-cost arsenic removal unit.
ALI, M. A. BADRUZZAMAN, A.B.M. JALIL, M.A. HOSSAIN, M. D. HUSSAINUZZAMAN, M. M. BADRUZZAMAN, M. MOHAMMAD, O.I. AKTER, N. (2001): Development of Low-cost Technologies for Removal of Arsenic from Groundwater. Dhaka: Bangladesh University of Engineering and Technology, Department of Civil Engineering URL [Accessed: 20.05.2019]Emerging and Innovative Techniques for Arsenic Removal Applied to a Small Water Supply System
This work presents a case study that describes the development of low-cost techniques for efficient arsenic control in drinking water. Iron oxide was used for arsenic removal and high removal efficiencies were monitored during the process of removal.
DUARTE A.A.L.S. ; CARDOSO, S.J.A. ; ALCADA, A.J. (2009): Emerging and Innovative Techniques for Arsenic Removal Applied to a Small Water Supply System. In: Sustainability: Volume 1 , 1288-1304. URL [Accessed: 20.05.2019]Arsenic Removal from Drinking Water During Coagulation
This paper presents the findings of a study on the efficiency of arsenic removal from source water and artificial freshwater during coagulation with ferric chloride and alum. They found that pH range for arsenic (V) removal with alum was more restricted than with ferric chloride and that arsenic (III) could not be removed from source water by coagulation with alum.
HERING, J.G. ; PEN-YUAN, C. ; WILKIE, J.A. ; ELIMELECH, M. (1997): Arsenic Removal from Drinking Water During Coagulation. In: Journal of Environmental Engineering: Volume 123 , 800-807. URL [Accessed: 14.05.2012]Safe Water Technology for Arsenic Removal. Technologies for arsenic Removal from Drinking Water
This paper describes some safe water technologies for arsenic removal, with special reference to experiences gained from field level application.
JOHNSTON, R. HEIJNEN, H. (2001): Safe Water Technology for Arsenic Removal. Technologies for arsenic Removal from Drinking Water. In: BANGLADESH UNIVERSITY OF ENGINEERING AND TECHNOLOGY ; UNITED NATIONS UNIVERSITY (2001): Technologies for Arsenic Removal from Drinking Water. Dhaka: 1-21. URL [Accessed: 14.05.2012]Removal of Copper, Chromium, and Arsenic by Water Hyacinths
The hyacinth’s tissues were analysed to evaluate the removal of copper (Cu), hexavalent chromium (Cr VI), and arsenic (As) from CCA (Chromated Copper Arsenate) contaminated water over a 17-day period. The results showed that the hyacinth was not a suitable plant to remediate arsenic and copper.
KEITH, C. BORAZJANI, H. DIEHL, S.V. SU, Y. BALDWIN, B.S. (2006): Removal of Copper, Chromium, and Arsenic by Water Hyacinths. (= Proceedings of the 36th Annual Mississippi Water Resources Conference, 25th to 26th April 2006 ). Jackson: Mississippi Water Resources Research Institute (MWRRI) URL [Accessed: 18.01.2011]High-Level Arsenite Removal from Groundwater by zerovalent Iron
The study assess the effectiveness of zerovalent iron for arsenic remediation in groundwater, determines removal mechanisms of arsenic and evaluates implications of these processes with regard to the stability of arsenic and long- term remedial performance of the permeable reactive barrier (PRB) technology.
LIEN, H.S. ; WILKIN, R.T. (2005): High-Level Arsenite Removal from Groundwater by zerovalent Iron. In: Chemosphere : Volume 59 , 377-386.Household Sand Filters for Arsenic Removal
Use of Arsenic Contaminated Sludge in Making Ornamental Bricks
This paper discusses the proper management and reuse of sludge generated during the treatment of arsenic contaminated water. It investigates the suitability of sludge in making brick. Results of different tests indicate the sludge proportion is the key factor for determining the quality of ornamental bricks/tiles. The study showed that arsenic contaminated sludge could be used safely up to a rate of 4% for making ornamental bricks.
MAHZUZ, H. M. A. ; ALAM, R. ; ALAM, M. M. ; BASAK, R. ; ISLAM, M.S. (2009): Use of Arsenic Contaminated Sludge in Making Ornamental Bricks. In: International Journal of Environmental Science and Technology 6: Volume 2 , 291-298. URL [Accessed: 20.05.2019]Low-cost Technique of Arsenic Removal from Water and its Removal Mechanism
This study examines the potential of removing arsenic from water by co-precipitation with naturally occurring iron. The experimental study examined the sensitivity of removal of arsenic in response to manual mixing and prolonged settlement.
MAMTAZ, R. BACHE, D.H. (2001): Low-cost Technique of Arsenic Removal from Water and its Removal Mechanism. In: BANGLADESH UNIVERSITY OF ENGINEERING AND TECHNOLOGY ; UNITED NATIONS UNIVERSITY (2001): Technologies for Arsenic Removal from Drinking Water. Dhaka: 43-58. URL [Accessed: 14.05.2012]Smart Disinfection Solutions
This booklet, part of the Smart Water Solutions series provides a wide range of methods and products for home water treatment in rural areas.
NWP (2010): Smart Disinfection Solutions. Examples of small-scale disinfection products for safe drinking water. (= Smart water solutions ). Amsterdam: KIT Publishers URL [Accessed: 17.05.2019]A Biomaterial Based Approach for Arsenic Removal from Water
In this paper, it is shown that a biomaterial produced from dried water hyacinths roots, a plant that is found in abundant supply in many parts of the world, can provide a simple, effective and yet cheap method for removing arsenic from contaminated water.
RMALLI, S. W. ; HARRINGTON, C. F. ; AYUB, M. ; HARIS, P. I. (2005): A Biomaterial Based Approach for Arsenic Removal from Water. In: Journal of Environmental Monitoring : Volume 7 , 279-282. URL [Accessed: 12.01.2011]Arsenic Removal Processes on Trial in Bangladesh
This paper reviews some of the arsenic removal technologies being tried and tested in Bangladesh.
SHARMIN, N. (2001): Arsenic Removal Processes on Trial in Bangladesh. In: BANGLADESH UNIVERSITY OF ENGINEERING AND TECHNOLOGY ; UNITED NATIONS UNIVERSITY (2001): Technologies for Arsenic Removal from Drinking Water. Dhaka: 23-30. URL [Accessed: 14.05.2012]Arsenic removal from drinking water using granular ferric hydroxide
This paper examines the use of granular ferric hydroxide to remove both arsenate and arsenite present in drinking water by conduction batch and column studies. Batch and column studies showed that granular ferric hydroxide (GFH) could be effectively used in small water utilities to achieve less than 5 μg As/l in drinking water.
THIRUNAVUKKARASU, O.S. ; VIRARAGHAVAN, T. ; SUBRAMANIAN, K.S. (2003): Arsenic removal from drinking water using granular ferric hydroxide. In: Water SA29: Volume 2 , 161-170. URL [Accessed: 18.01.2011]Review of Arsenic Removal Technologies for Contaminated Groundwater
This article summarises the technologies currently being investigated to remove arsenic from drinking waters, with a special focus on developing and third-world countries where the problem is exacerbated by flooding and depressed economic conditions.
VU, K.B. KAMINSKI, M.D. NUNEZ, L. (2009): Review of Arsenic Removal Technologies for Contaminated Groundwater. Argonne: University of Chicago - Argonne National Laboratory URL [Accessed: 12.01.2011]SORAS - a simple arsenic removal process
This paper gives an overview on SORAS (Solar oxidation and removal of arsenic) with laboratory and field test results.
WEGELIN, M. GECHTER, D. HUG, S. MAHMUD, A. MOTALEB, A. (1999): SORAS - a simple arsenic removal process. Duebendorf: Eawag/SANDEC URL [Accessed: 20.05.2019]Conservation et Traitement de l Eau a Domicile
This practical guide provides a review of different processing techniques and adequate water conservation at home and is structured around 10 key questions that should be posed before choosing a suitable solution.
DESILLE, D. (2013): Conservation et Traitement de l Eau a Domicile. Paris: Programme Solidarite Eau (PSeau) URL [Accessed: 06.06.2013]Predicting Water Consumption Habits for Seven Arsenic-Safe Water Options in Bangladesh
In Bangladesh, 20 million people are at the risk of developing arsenicosis because of excessive arsenic intake. Despite increased awareness, many of the implemented arsenic-safe water options are not being sufficiently used by the population. This study investigated the role of social-cognitive factors in explaining the habitual use of arsenic-safe water options.
INAUEN, J. ; TOBIAS, R. ; MOSLER, H.-J. (2013): Predicting Water Consumption Habits for Seven Arsenic-Safe Water Options in Bangladesh. In: BMC Public Health: Volume 13 , 1-10. URL [Accessed: 28.08.2013]An Overview of Arsenic Removal Technologies in Bangladesh and India
The most common technologies for arsenic removal utilise conventional processes like oxidation, co-precipitation, adsorption, ion exchange and membrane techniques. The paper gives a short review of all these technologies, which are used in Bangladesh and India.
AHMED, M. F. (2001): An Overview of Arsenic Removal Technologies in Bangladesh and India. In: BANGLADESH UNIVERSITY OF ENGINEERING AND TECHNOLOGY ; UNITED NATIONS UNIVERSITY (2001): Technologies for Arsenic Removal from Drinking Water. Dhaka: 251-269. URL [Accessed: 21.05.2019]Risk Assessment of Arsenic Mitigation Options in Bangladesh
This study assesses water quality and sanitary conditions to estimate the burden of disease associated with each technology in disability adjusted life years (DALYs). The findings suggest that deep tube wells and rain harvesting provide safe water.
HOWARD, G. ; AHMED, M.F. ; SHAMSUDDIN, A.J. ; MAHMUD, S.G. ; DEERE, D. (2006): Risk Assessment of Arsenic Mitigation Options in Bangladesh. In: Journal of Health Population and Nutrition 24: Volume 3 , 346-355. URL [Accessed: 18.01.2011]Evaluation of Three Arsenic Removal Technologies in Nepal
The three arsenic removal technologies (Three-Gagri System, the Jerry Can System, and the Arsenic Treatment Unit (ATU)) were evaluated for their effectiveness and appropriateness.
HURD, J.J. (2001): Evaluation of Three Arsenic Removal Technologies in Nepal. (= Master-Thesis ). Los Angeles: University of California URL [Accessed: 20.05.2019]Arsenic Removal from Groundwater Containing Iron, Ammonium, Manganese and Phosphate: A Case Study from a Treatment Unit in Northern Greece
This paper presents the application of treatment method for the removal of iron, manganese, ammonium and arsenic from groundwater. The research was carried out in Northern Greece in the city of Malgara.
KATSOYIANNIS, I.A. ; ZIKOUDI, A. ; HUG, S.J. (2008): Arsenic Removal from Groundwater Containing Iron, Ammonium, Manganese and Phosphate: A Case Study from a Treatment Unit in Northern Greece. In: Desalination : Volume 224 , 330-339. URL [Accessed: 14.05.2012]The Arsenic Biosand Filter project: Design of an Appropriate Household Drinking Water Filter For Rural Nepal - Final Report
This study evaluates the performance of the filter under various set-ups, to investigate long-term removal efficiencies to improve the filter design and to implement the filter in arsenic affected villages.
NGAI, T. WALEWIJK, S. (2003): The Arsenic Biosand Filter project: Design of an Appropriate Household Drinking Water Filter For Rural Nepal - Final Report. Cambridge: Massachusetts Institute of Technology URL [Accessed: 18.01.2011]Appraisal of Two Indigenous Household Groundwater Arsenic Removal Technologies for Bangladesh under Field Conditions
This paper elaborates on the performance of two indigenous arsenic (As) removal techniques, theSafi filter and home-based filters (Chari/Pitcher filter). The filters were developed to serve household drinking water requirementsand were evaluated at field conditions and compared with those reported in literature.
RAHMAN, I.M.M. ; HOSSAIN, M.M. ; HELAL UDDIN, M. ; NAZIMUDDIN, M. ; MAJD, M.A. (2005): Appraisal of Two Indigenous Household Groundwater Arsenic Removal Technologies for Bangladesh under Field Conditions. In: Journal of Agriculture and Social Sciences: Volume 4 , 361-365. URL [Accessed: 20.05.2019]Review of Coagulation Technology for Removal of Arsenic: Case of Chile
This paper presents a summary of the process, concepts and operational considerations for the use of coagulation technology for removal of arsenic in Chile.
SANCHA, A. M. (2006): Review of Coagulation Technology for Removal of Arsenic: Case of Chile. In: Journal of Health, Population and Nutrition: Volume 24 , 267-272. URL [Accessed: 14.05.2012]Technical and Social Evaluation of Arsenic Mitigation in Rural BangladeshChile
This paper presents the findings of technical and social performances of the SONO Arsenic Filter, an arsenic removal technology in rural areas of Bangladesh.
SHAFIQUZZAMAN, M. ; AZAM, M.S. ; MISHIMA, I. ; NAKAJIMA, J. (2009): Technical and Social Evaluation of Arsenic Mitigation in Rural BangladeshChile. In: Journal of Health Population and Nutrition 27: Volume 5 , 674-683. URL [Accessed: 18.01.2011]Rapid Assessment of Technologies for Arsenic Removal at the Household Level
This paper reviews the results and conclusions from a DFID-funded project ‘Rapid Assessment of Household Level Arsenic Removal Technologies’ carried out in association with the Bangladesh Arsenic Mitigation Water Supply Project. Nine technologies were assessed under this project.
SUTHERLAND, D. KABIR, M.O. CHOWDHURY, N.A. (2001): Rapid Assessment of Technologies for Arsenic Removal at the Household Level. In: BANGLADESH UNIVERSITY OF ENGINEERING AND TECHNOLOGY ; UNITED NATIONS UNIVERSITY (2001): Technologies for Arsenic Removal from Drinking Water. Dhaka: 190-200. URL [Accessed: 14.05.2012]Arsenic Removal Technologies for Drinking Water in Vietnam
This study presents laboratory and field test results and assesses the suitability of using oxidation processes by activated hypochlorite in water treatment plants in Hanoi city and naturally occurring minerals as sorbents in household-based systems to reduce arsenic concentrations in drinking water.
VIET, P.H. CON, T.H. HA, C.T. TIN, N.V. BERG, M. GIGER, W. SCHERTENLEIB, R. (n.y): Arsenic Removal Technologies for Drinking Water in Vietnam. Bangkok: UNESCAP URL [Accessed: 14.05.2012]Arsenic Contamination in Southeast Asia Region: Technologies for Arsenic Mitigation
PowerPoint presentation in PDF version, which includes information on arsenic contamination globally and in Southeast Asia with an overview on existing arsenic mitigation water supply technologies.
AHMED, M. F. (n.y): Arsenic Contamination in Southeast Asia Region: Technologies for Arsenic Mitigation. (= PPT Presentation ). Dhaka: Bangladesh University of Engineering and Technology URL [Accessed: 14.05.2012]An Introduction to Household Water Treatment and Safe Storage, A CAWST Training Manual
This training manual describes the need of safe drinking water and sanitation and provides relevant information on HWTS process, technologies. It is good reference material for trainers to conduct training on HWTS.
CAWST (2009): An Introduction to Household Water Treatment and Safe Storage, A CAWST Training Manual. Calgary: Centre for Affordable Water and Sanitation Technology (CAWST) URL [Accessed: 13.05.2019]Arsenic Removal. Adsorption
Factsheet on the principles, construction, operation and maintenance of arsenic removal via adsorption for drinking water treatment in developing countries.
CAWST (2009): Arsenic Removal. Adsorption. (= Household Water Treatment and Safe Storage Fact Sheet - Academic ). Centre for Affordable Water and Sanitation Technology (CAWST) URL [Accessed: 20.05.2019]Arsenic Removal. Complexation
Factsheet on the principles, construction, operation and maintenance of arsenic removal via complexation for drinking water treatment at household level.
CAWST (2009): Arsenic Removal. Complexation. (= Household Water Treatment and Safe Storage Fact Sheet - Academic ). Centre for Affordable Water and Sanitation Technology (CAWST) URL [Accessed: 21.05.2019]Arsenic Removal. Oxidation
Factsheet on the principles, construction, operation and maintenance of arsenic removal via oxidation for drinking water treatment at household level.
CAWST (2009): Arsenic Removal. Oxidation. (= Household Water Treatment and Safe Storage Fact Sheet - Academic ). Centre for Affordable Water and Sanitation Technology (CAWST) URL [Accessed: 21.05.2019]Kanchan Filter. Fact Sheet - Academic
Factsheet on the principles, construction, operation and maintenance of kanchan filters (for arsenic and pathogen removal) for drinking water treatment at household level.
CAWST (2009): Kanchan Filter. Fact Sheet - Academic. (= Household Water Treatment and Safe Storage Fact Sheet - Academic ). Centre for Affordable Water and Sanitation Technology (CAWST) URL [Accessed: 21.05.2019]Kanchan Filter. Fact Sheet - Simplified
Simplified factsheet on the principles, construction, operation and maintenance of Kanchan filters (for arsenic and pathogen removal) for drinking water treatment in at the household level.
CAWST (2009): Kanchan Filter. Fact Sheet - Simplified. (= Household Water Treatment and Safe Storage Fact Sheet - Simplified ). Centre for Affordable Water and Sanitation Technology (CAWST) URL [Accessed: 21.05.2019]Arsenic Poisoning and the Kanchan Arsenic Filter
Video produced by Global Water Trust (2008), showing arsenic scenarios and the implementation of KanchanTM Arsenic Filter.
Global Water and Sanitation Projects
This weblink provides information on WATSAN and researches on Household water treatment technologies including KanchanTM Arsenic Filter by MIT.
WHO - Arsenic
This web link contains a general discussion on different arsenic removal options for controlling risk.
Arsenic: Coagulatin/Filtration Processes
This web link connects to a video on arsenic treatment by coagulation/filtration processes.

